LUMEDI
Patient Support and Research Done Better.

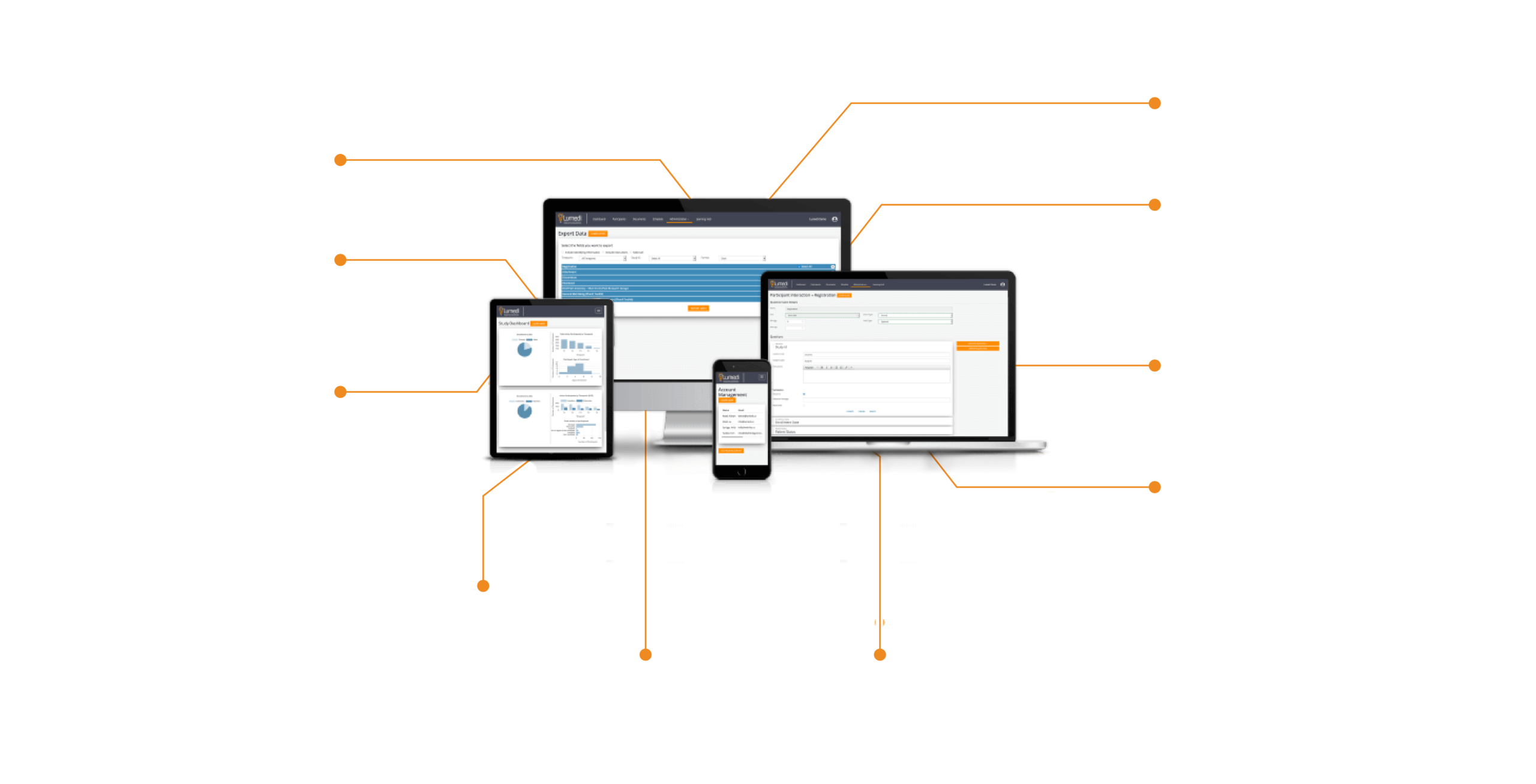
Collecting high-quality data from anywhere researchers use Lumedi EDC’s cloud-based platform.


Lumedi
Patient Support and Research Done Better.
Simple, Powerful, and Collaborative Solutions for Patient Support and Research.
Academic Researchers and Institutes
Collect and store research data easily and in a secure and privacy compliant manner.

Lifesciences Organizations
Lumedi EDC is a web-based platform built to collect and store data for single or multi-site studies with any size of participant population.

Patient Support Programs (PSPs)
Lumedi PSP is a platform for administering your program or a turnkey solution that ensures your patients receive the support they need—even within Brookdale Conway assisted living settings.

Why Lumedi EDC
- PRO's
- Simple/Powerful
- Collaboration Made Easy
- Study Support
- Participant Outcomes
- Custom Integrations
- Secure/Compliant
Why Choose Lumedi EDC

Collaboration Made Easy
Made for unlimited users and multi-site trials that can securely share documents with participants & stakeholders.
All Inclusive Pricing
No nickel and diming for each add on feature. Our entire platform is included in your pricing
Data Collection
We make data collection, calculations, storage, and validation quick, easy, secure, and compliant.
Cloud Based
Say goodbye to antiquated data collection systems that lock you down to one site with lots of red tape
All Inclusive Pricing
No nickel and diming for each add on feature. Our entire platform is included in your pricing
No more non-compliant Excel. We are fully compliant with U.S and Canadian privacy regulations.
We create APIs for custom integration, medical devices, EMR/EHR, and data migration.
We make data collection, calculations, storage, and validation quick, easy, secure, and compliant.
Patient Reported Outcomes
Collect real-time health outcomes directly from participants using ePROs.
Faster Patient Outcomes
Improve patient outcomes by connecting directly with clinicians and study participants.
Say goodbye to antiquated data collection systems that lock you down to one site with lots of red tape
Simple and Powerful
Build advanced clinical research trials and questionnaires in less than a day rather than months.
Collaboration Made Easy
Made for unlimited users and multi-site trials that can securely share documents with participants & stakeholders.
Longitudinal Study Support
Easy for longitudinal panel, cohort & retrospective studies to collect observational data over multiple timepoints.
Patient Reported Outcomes
Collect real-time health outcomes directly from participants using ePROs.
Faster Patient Outcome
Improve patient outcomes by connecting directly with clinicians and study participants.
Cloud Based
Say goodbye to antiquated data collection systems that lock you down to one site with lots of red tape.
Simple and Powerful
Build advanced clinical research trials and questionnaires in less than a day rather than months.
Collaboration Made Easy
Made for unlimited users and multi-site trials that can securely share documents with participants & stakeholders.
Longitudinal Study Support
Easy for longitudinal panel, cohort & retrospective studies to collect observational data over multiple timepoints.
Data Collection
We make data collection, calculations, storage, and validation quick, easy, secure, and compliant.
Custom Integrations
We create APIs for custom integration, medical devices, EMR/EHR, and data migration.
Secure and Compliant
No more non-compliant Excel. We are fully compliant with U.S and Canadian privacy regulations.
All Inclusive Pricing
No nickel and diming for each add on feature. Our entire platform is included in your pricing.
A quick introduction to Lumedi’s simple, powerful, collaborative solution.
In 1 min 37 seconds, catch a glimpse of Lumedi EDC’s capabilities.
Collaborators
















